Polarity. Polar and non-polar solvents and adsorbents
October 3, 2023
How to start working with a new reversed-phase column?
October 18, 2023What do “normal phase” and “reversed phase” mean?
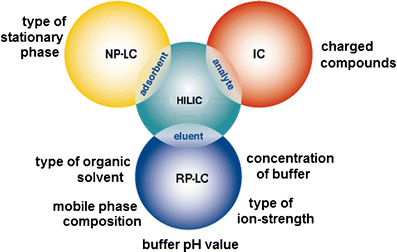
In liquid chromatography, the adsorbate interacts with both the adsorbent and the eluent. As a result, retention is determined by the difference between the two interactions.
The moral is: in liquid chromatography, we can always compensate for an adsorbate-adsorbent interaction with another adsorbate-eluent interaction. Moreover, exactly to the extent that we need for acceptable retention of the target compound. This is exactly how the retention time of liquid chromatography is regulated - by selecting the composition of the eluent, which achieves a good balance of two forces, two interactions.
Chromatographers jokingly call this principle “hold and release.” A correctly selected eluent should neither abruptly wash away the substance from the adsorbent nor leave it on the adsorbent. Everything should be washed off, but gradually.
First, let's find out: which solvent mixtures are used to prepare eluents in normal-phase chromatography, and which ones in reverse-phase chromatography?
So, in reverse phase chromatography the adsorbent is non-polar; The eluent is based on a polar solvent, which is mixed in a suitable proportion with a non-polar additive. The higher the proportion of non-polar additive in the eluent, the lower the retention.
The most common type of adsorbent for RP HPLC is C18 silica gel. The most common eluent base is water or water-salt buffer. Acetonitrile and methanol are often used as non-polar additives, less often tetrahydrofuran or isopropyl alcohol, which have a high elution force.
Now about normal phase chromatography. Speaking very superficially, here everything is the other way around with respect to the OF option.
The base is a less polar solvent than the additive. The additive this time is called polar additive. The higher the proportion of polar additive in the eluent, the lower the retention of substances. The most common polar adsorbent for NF HPLC is silica gel.
Further - more difficult. Normal-phase systems are divided into “classical” normal-phase and hydrophilic (H ILIC, hydrophilic interaction chromatography).
In general, the basis of the eluent in “classical” normal-phase chromatography, as a rule, is hexane. Many solvents can be used as a polar additive: ethers (dioxane, tetrahydrofuran), esters (ethyl acetate), alkyl chlorides (chloroform, methylene chloride), ketones (acetone), often alcohols (especially isopropyl alcohol, which is indefinitely miscible with hexane).